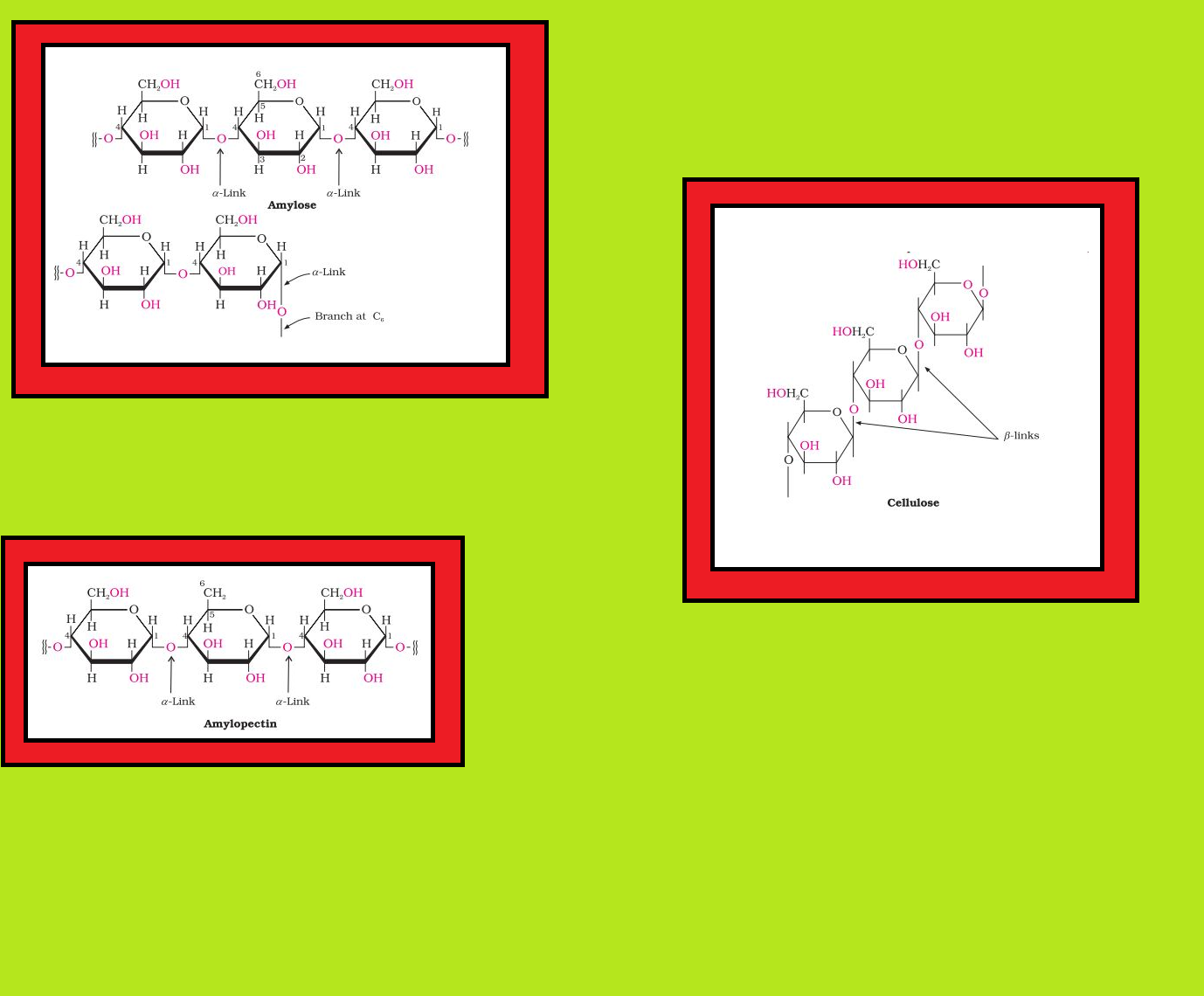
Disaccharides :
`=>` Disaccharides on hydrolysis with dilute acids or enzymes yield two molecules of either the same or different monosaccharides.
● The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through oxygen atom is called `color{green}("glycosidic linkage")`.
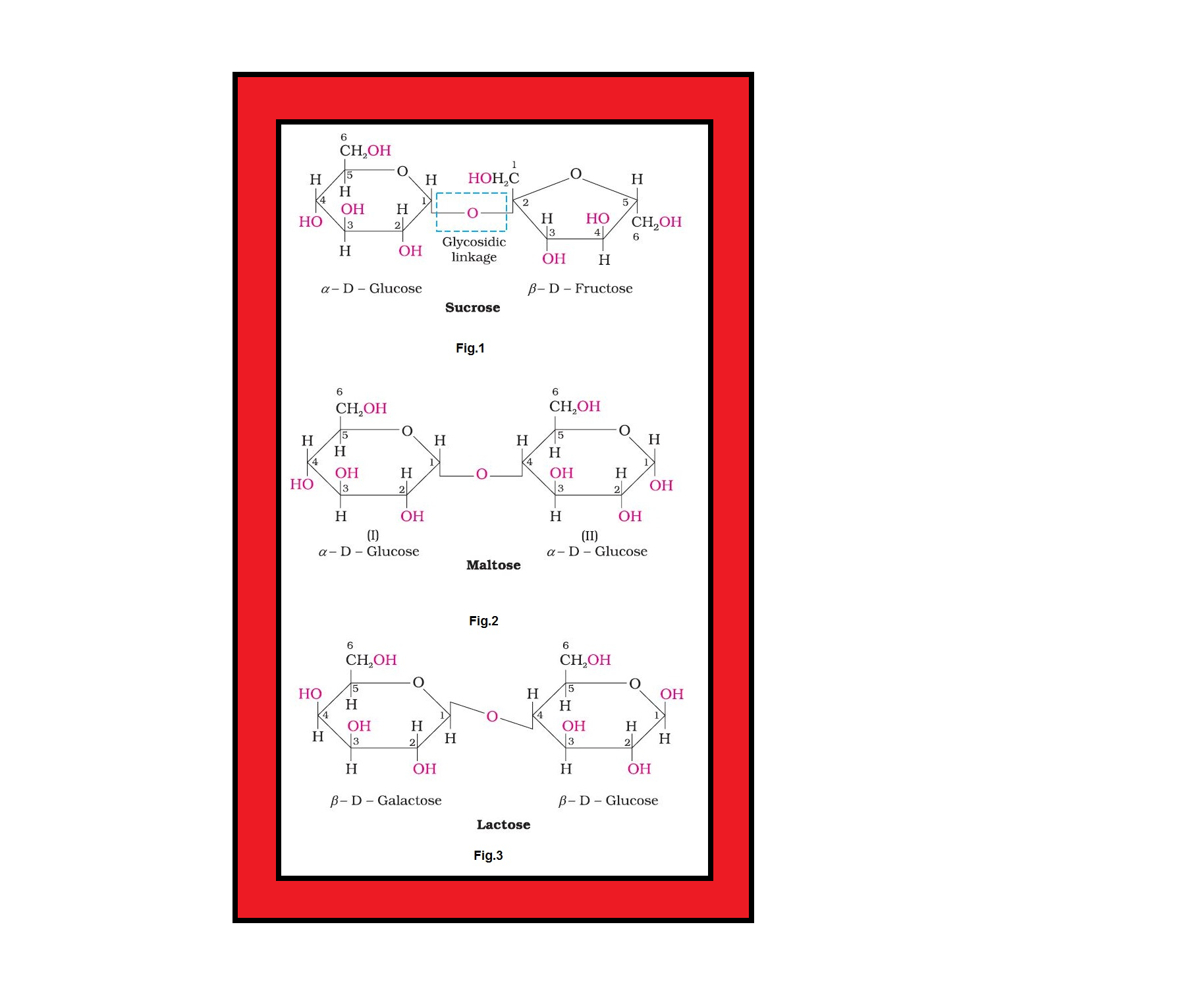
(i) `color{green}("Sucrose ")` : One of the common disaccharides is sucrose which on hydrolysis gives equimolar mixture of `color{red}(D-(+))`-glucose and `color{red}(D-(-))` fructose.
`color{red}(undersettext(Sucrose)(C_(12)H_(22) O_(11)) +H_2O → undersettext{D- (+) - Glucose} (C_6H_(12)O_6) + undersettext{D-(-)-Fructose} (C_6H_(12)O_6))`
● These two monosaccharides are held together by a glycosidic linkage between `color{red}(C-1)` of `color{red}(α)`-glucose and `color{red}(C-2)` of `color{red}(β)`-fructose. See fig.1.
● Since the reducing groups of glucose and fructose are involved in glycosidic bond formation, sucrose is a non reducing sugar.
● Sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose.
● Since the laevorotation of fructose `(–92.4°)` is more than dextrorotation of glucose `(+ 52.5°)`, the mixture is laevorotatory.
● Thus, hydrolysis of sucrose brings about a change in the sign of rotation, from dextro `color{red}((+))` to laevo `color{red}((–))` and the product is named as `color{green}("invert sugar")`.
(ii) `color{green}("Maltose ")` : Another disaccharide, maltose is composed of two `color{red}(α-D)`-glucose units in which `color{red}(C-1)` of one glucose `color{red}((I))` is linked to `color{red}(C-4)` of another glucose unit (II). See fig.2.
● The free aldehyde group can be produced at `color{red}(C-1)` of second glucose in solution and it shows reducing properties so it is a reducing sugar.
(iii) `color{green}("Lactose ")` : It is more commonly known as `color{green}("milk sugar")` since this disaccharide is found in milk.
● It is composed of `color{red}(β-D)`-galactose and `color{red}(β-D)`-glucose.
● The linkage is between `color{red}(C-1)` of galactose and `color{red}(C-4)` of glucose. Hence it is also a reducing sugar. See fig.3.
● The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through oxygen atom is called `color{green}("glycosidic linkage")`.
(i) `color{green}("Sucrose ")` : One of the common disaccharides is sucrose which on hydrolysis gives equimolar mixture of `color{red}(D-(+))`-glucose and `color{red}(D-(-))` fructose.
`color{red}(undersettext(Sucrose)(C_(12)H_(22) O_(11)) +H_2O → undersettext{D- (+) - Glucose} (C_6H_(12)O_6) + undersettext{D-(-)-Fructose} (C_6H_(12)O_6))`
● These two monosaccharides are held together by a glycosidic linkage between `color{red}(C-1)` of `color{red}(α)`-glucose and `color{red}(C-2)` of `color{red}(β)`-fructose. See fig.1.
● Since the reducing groups of glucose and fructose are involved in glycosidic bond formation, sucrose is a non reducing sugar.
● Sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose.
● Since the laevorotation of fructose `(–92.4°)` is more than dextrorotation of glucose `(+ 52.5°)`, the mixture is laevorotatory.
● Thus, hydrolysis of sucrose brings about a change in the sign of rotation, from dextro `color{red}((+))` to laevo `color{red}((–))` and the product is named as `color{green}("invert sugar")`.
(ii) `color{green}("Maltose ")` : Another disaccharide, maltose is composed of two `color{red}(α-D)`-glucose units in which `color{red}(C-1)` of one glucose `color{red}((I))` is linked to `color{red}(C-4)` of another glucose unit (II). See fig.2.
● The free aldehyde group can be produced at `color{red}(C-1)` of second glucose in solution and it shows reducing properties so it is a reducing sugar.
(iii) `color{green}("Lactose ")` : It is more commonly known as `color{green}("milk sugar")` since this disaccharide is found in milk.
● It is composed of `color{red}(β-D)`-galactose and `color{red}(β-D)`-glucose.
● The linkage is between `color{red}(C-1)` of galactose and `color{red}(C-4)` of glucose. Hence it is also a reducing sugar. See fig.3.